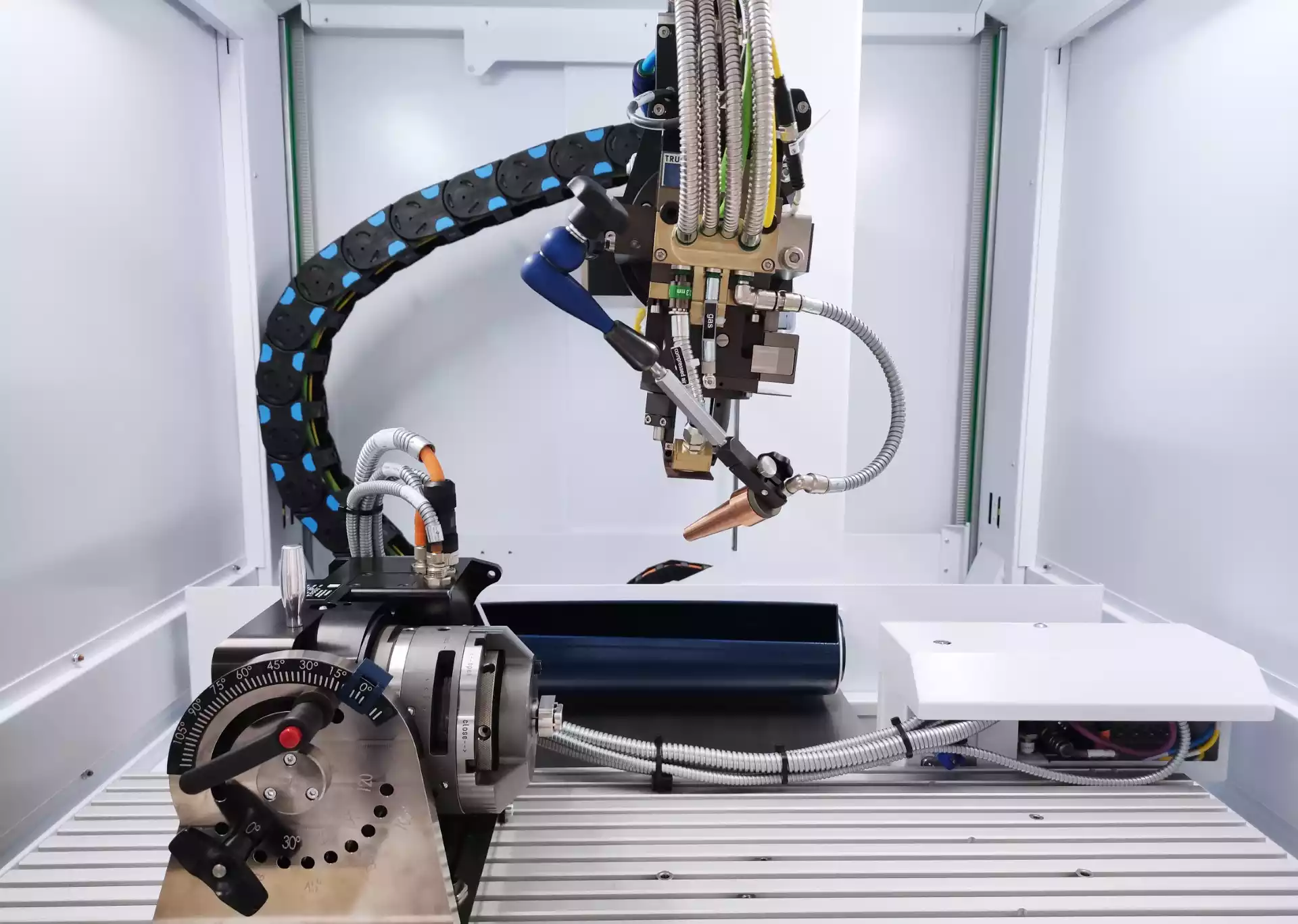
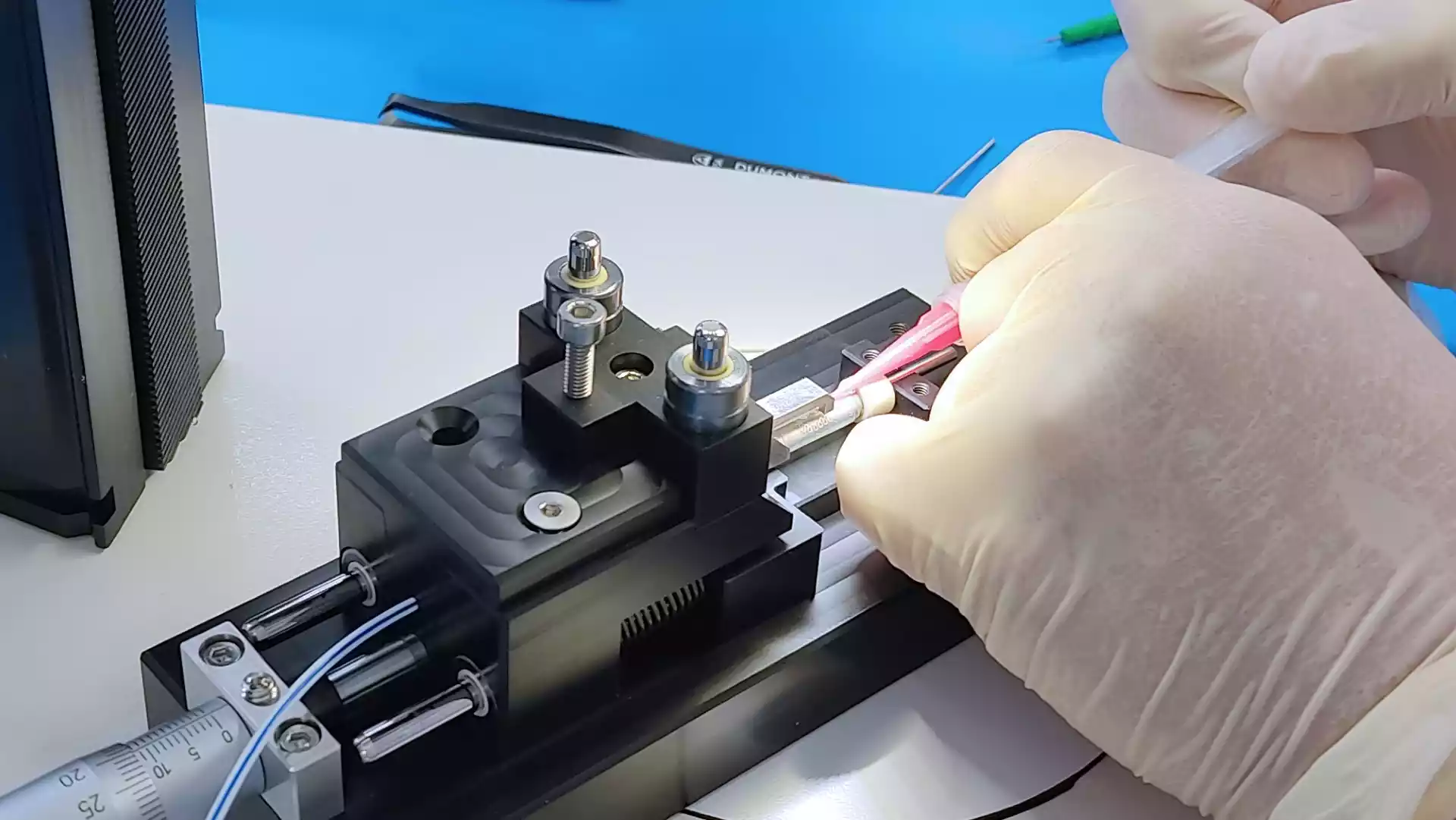
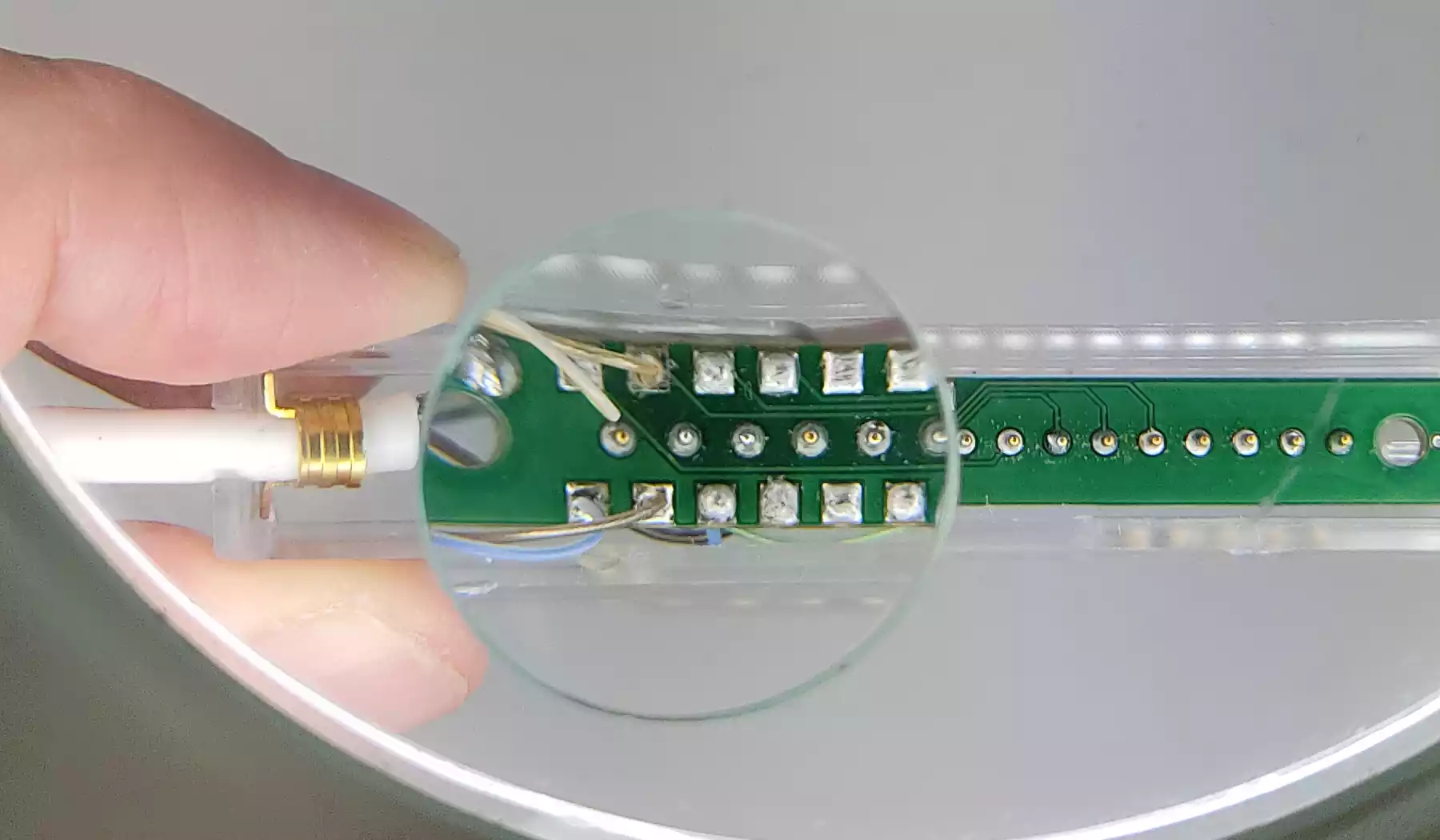
Our team helps you to make the transition from R&D to manufacturing. We challenge design choices to ensure that the device is manufacturable, but also respects set cost targets.
The design for manufacturing phase enables us to integrate manufacturing constraints as design input data.
Begun as early as possible, this phase enables the design to evolve before a design freeze, which can be restrictive in terms of cost.