
We are delighted to announce the renewal of our NF EN ISO 13485 : 2016 certification.
We have also extended our scope of certification to include design, development and manufacturing of :
-
Active implantable medical devices
-
Electro-medical devices integrating electronics and embedded software
Already existing within Cisteo MEDICAL, these services are at the heart of the solutions we aim to provide to medtech start-ups.
Our quality management system has undergone major changes, while preserving coherence and conformity praised by our auditors.
A single service provider to simplify the development of your medical device (from R&D to series production)
By this move, Cisteo MEDICAL is renewing its commitment to medtech start-ups, and underscoring our desire to be a single point of contact to:
-
Providing specialized expertise and technical skills in line with regulatory requirements
-
Providing scalable and sufficient production capacity in line with volume increases
Growing expertise in electro-medical devices
 Thanks to a multidisciplinary team, Cisteo MEDICAL are now able to handle R&D, design and manufacturing phases of electro-medical devices integrating electronics and embedded software (class A, B and C).
Thanks to a multidisciplinary team, Cisteo MEDICAL are now able to handle R&D, design and manufacturing phases of electro-medical devices integrating electronics and embedded software (class A, B and C).Software development is carried out in compliance with the IEC 62304 reference standard, and we take the normative context into consideration from the earliest design stages, in order to propose a prototype and/or proof-of-concept that complies:
-
Electronics-relateds: this takes the form of component selection and compliance with applicable standards (Standard 60601-1 and collateral standards).
-
Software-related : we focus in particular on writing and documenting the software life cycle.
Three aspects have been put in place to provide these services in the best possible conditions:
-
Training in essential requirements
-
Documentation
-
Resources
Expertise in medical devices up to class III and active implants
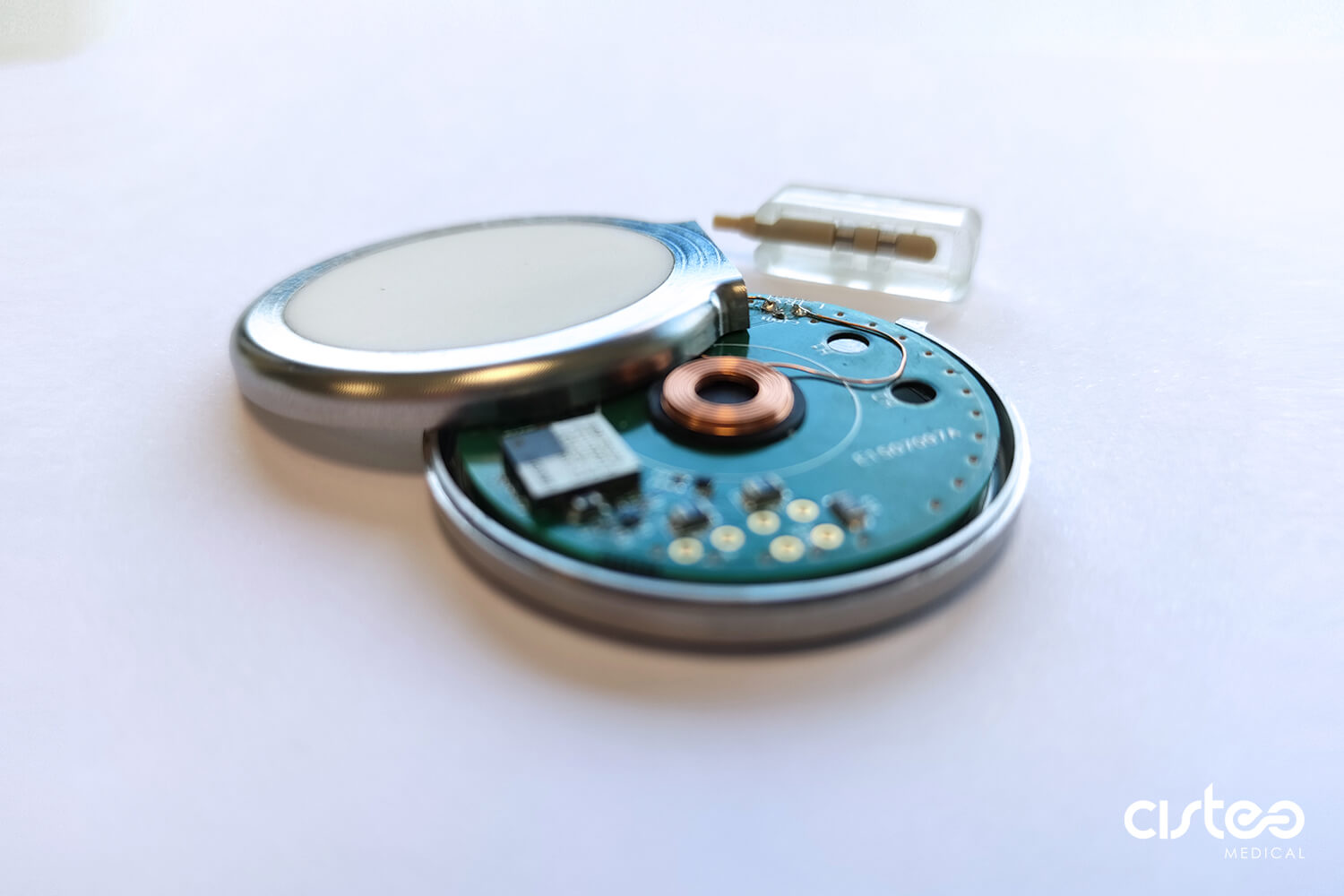 The certificate now includes the concept of active implantable devices. These skills have already been integrated within the company, and are now recognized from a normative angle.
The certificate now includes the concept of active implantable devices. These skills have already been integrated within the company, and are now recognized from a normative angle.Following R&D and design stages, Cisteo MEDICAL follows the developments of start-ups for which we are working, and providing services such as industrialisation and sub-contract manufacturing of electro-medical devices.
Thanks to these different developments, we are keen on building a long-term partnership based on the following stages:
- Co-design between electronics, mechanics and design teams
- Industrialisation
- Assembly, mounting, electrical wiring, inspection and testing
Growing production capacity to match the needs of medtech start-ups
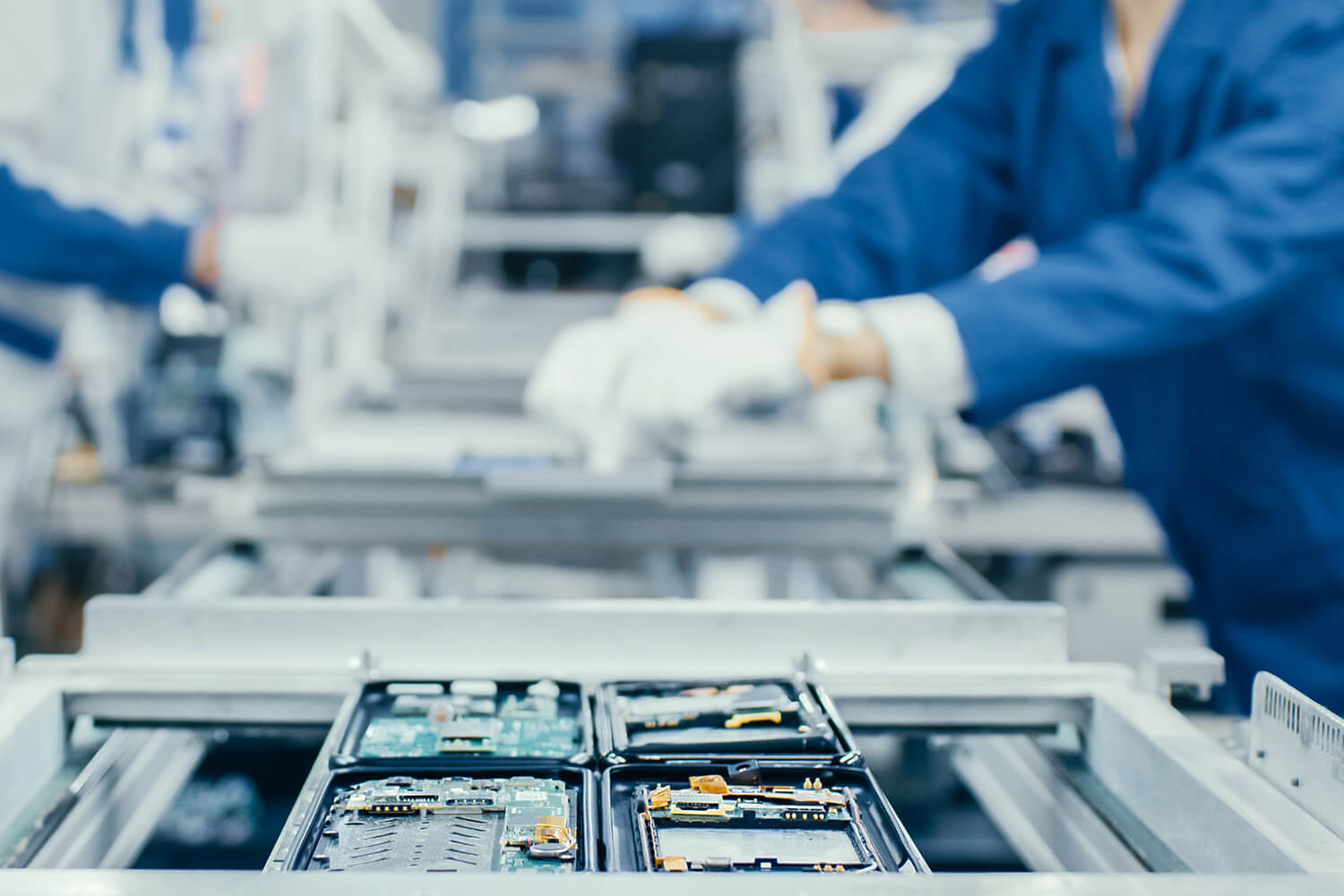 This certificate renewal also see the certification of a new manufacturing plant.
This certificate renewal also see the certification of a new manufacturing plant. Based just about hundred meters from our main site, this facility houses an area specifically designed for the assembly and wiring of electro-medical machines. This site also offers the possibility of dedicating an area for specific manufacturing, a flexibility appreciated in scale-up stages.
The new site also incorporates a cleanroom configured to ISO7 requirements, providing our manufacturing customers with additional capacity.
Interested in growing your company together?
We'll be happy to discuss your needs and requirements with you. Please fill in the form below, and we'll connect you with the right department.
