Covering every aspect of the development of your medical devices, our team of 45 employees (technicians, engineers or PhDs) stands out for its expertise, experience and appetite for medical innovation.


Specializing in the development, manufacture and approval of medical devices under contract, Cisteo MEDICAL supports you at every stage of your project. From the first thoughts to production in a controlled atmosphere, via design verification and regulatory procedures, we bring your ideas to life in various fields such as minimally invasive surgery (implants and instrumentation), but also cardiac surgery, cardiology, spinal surgery, ENT, obesity treatment, urology, and so on.
Based in Besançon, our medical device company benefits from the experience of the microtechnology capital and the stimulating environment of the research centers in Burgundy-Franche-Comté. Cisteo MEDICAL's core business and our company's DNA, medical innovation means that we work particularly for medtech start-ups and spin-offs (90%), as well as for healthcare professionals.
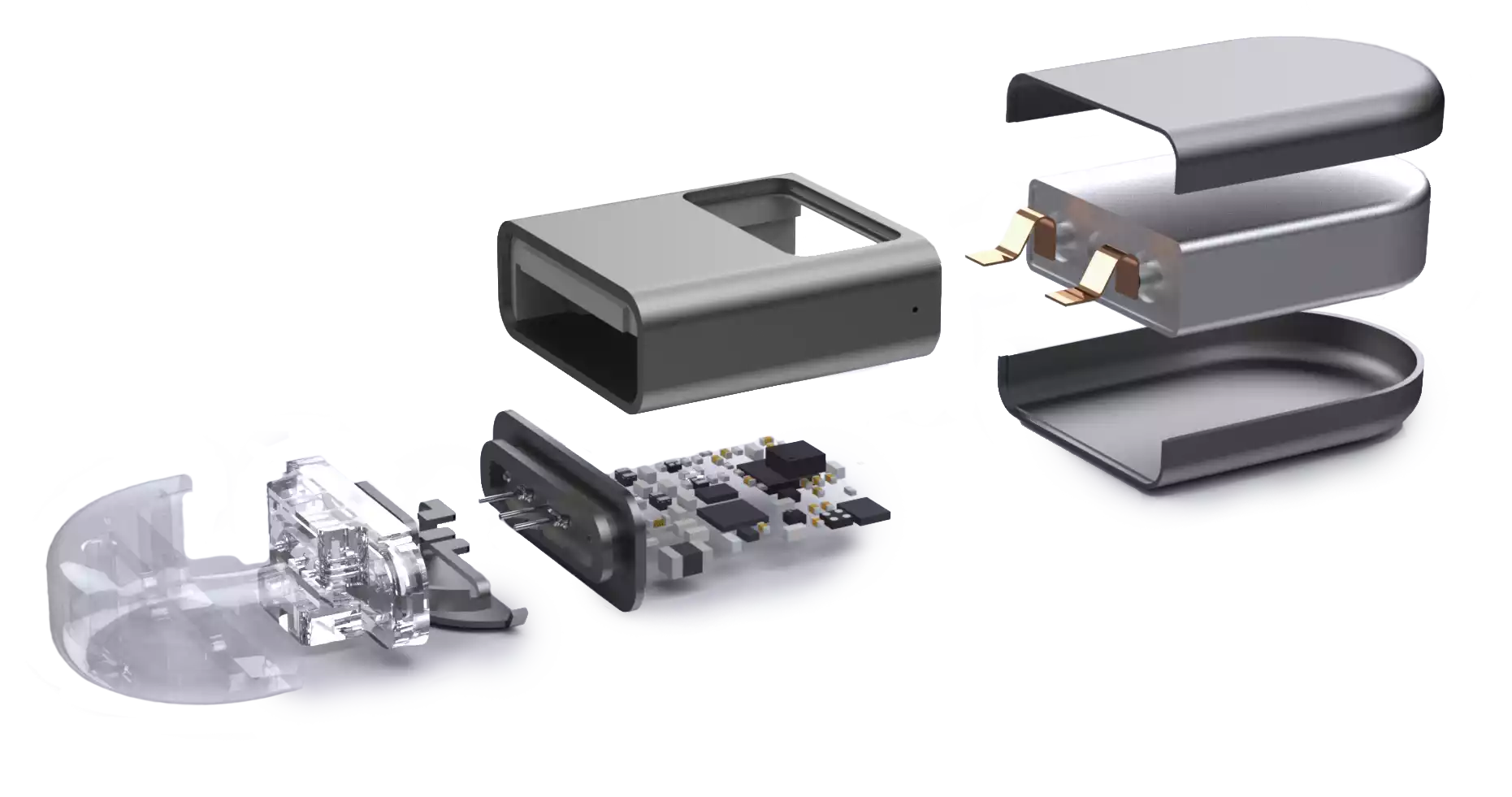
With over 15 years' experience in the research and development of class III medical devices alongside start-ups, spin-offs and manufacturers, our team brings you its best technical, microtechnical and electronic solutions. Our manufacturing and assembly services for medical devices in controlled environments enable us to bring all your projects to fruition: silicone transformation, nitinol shaping, 5-axis laser welding, laser marking, heat treatment, cleaning, packaging...
In addition, our regulatory and standards support ensures the conformity of your medical devices. Approved for Research Tax Credit (CIR) and Innovation Tax Credit (CII) by the French Ministry of Higher Education and Research, Cisteo MEDICAL provides the documentation you need to build your CE marking or FDAmarki file, and helps you create and implement your ISO 13485 and/or 21 CFR part 820quality management system.

With Cisteo MEDICAL, we don't just design and manufacture your device, but also offer you comprehensive and/or targeted support, depending on your needs.